One Health – What is it?
One Health is defined by the American Veterinary Medical Association (AVMA) as “the integrative effort of multiple disciplines working locally, nationally, and globally to attain optimal health for people, animals, and the environment” (AVMA, 2018). The health of people, animals, and the environment are inextricably linked. The problem of antimicrobial resistance (AMR) is a prime example of the importance of One Health since antibiotic use in any one of these areas can potentially impact the health of the others. For example, AMR fostered by drug use in animals can negatively impact human well-being and vice versa. Additionally, antibiotics administered to both people and animals inevitably end up in the environment which can simultaneously impact ecosystem health and potentially become a reservoir for resistant organisms. In order to mitigate the emergence and spread of AMR and preserve antimicrobial efficacy, trained professionals (e.g., veterinarians, physicians, ecologists, and agricultural professionals) must work collaboratively to ensure that antimicrobial products are used judiciously. This learning module will discuss using the One Health approach to curb the increasing prevalence of AMR both locally and globally.
While many microorganisms (i.e., microscopic organisms like bacteria, fungi, parasites, and protozoa) can become resistant to antimicrobial drugs, this module focuses on antimicrobial resistance in bacteria. [See the Pharmacology Module for further information about “antimicrobial” terminology (Antimicrobial Drugs: An Introduction)].
LEARNING OUTCOMES
This module provides an overview of the One Health approach and why it is key to addressing the problem of antimicrobial resistance. By the end of this module, you will be able to:
- Discuss One Health and its importance in antimicrobial resistance.
- Understand the impact of imprudent or non-judicious antimicrobial drug use.
- Explain how the One Health approach can mitigate AMR emergence and transmission.
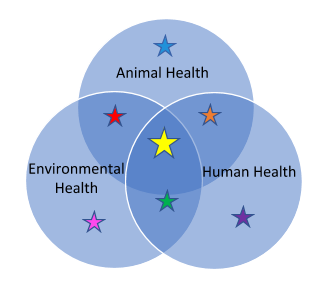
This One Health figure will help guide you through this module. The colored stars match with corresponding sections.
One Health and Antimicrobial Resistance
One Health and Antimicrobial Resistance
Antimicrobial resistance (AMR) is the ability of microbes like bacteria to resist the effects of an antimicrobial drug. In other words, the bacteria are either not killed or their growth is not stopped by the antimicrobial drug(s). Antimicrobial resistance is a natural phenomenon—all bacteria can evolve to develop resistance (see the Microbiology Module to learn more about bacterial resistance strategies and molecular mechanisms of resistance)—but any antibiotic use, anywhere bacteria can be found (in humans, animals, or in the environment), also can contribute to the development of resistance. Inappropriate antibiotic use in any sector is a concerning driver of resistance and is something that requires a One Health approach in order to address resistance effectively. Specifically, the problem of antimicrobial resistance cannot be solved by addressing antimicrobial use in only one sector.
Controlling and preventing AMR has become a public health priority as reports of AMR emergence and spread are increasing around the world (see the Antimicrobial Resistance: Global Report on Surveillance 2014 and the Global Antimicrobial Resistance Surveillance System (GLASS)). One option to combat AMR is to work towards discovering new antimicrobial drugs and alternative therapies against infections. However, given the significant amount of time and expense required to develop new treatments and the critical (often life-saving) role that antibiotics currently play in our daily lives, it is important to preserve the efficacy of existing antibiotics by promoting appropriate use and reducing inappropriate use.
One way to apply the One Health approach is to develop mitigation and educational tools around antibiotic stewardship (see Antimicrobial Stewardship box below). Since antimicrobial resistance can evolve naturally and keep re-emerging, eradication of all drug resistance is not feasible. The One Health concept provides an avenue for ongoing response (Wellcome Trust, 2017).
|
ANTIMICROBIAL STEWARDSHIP The American Veterinary Medical Association (AVMA) has defined antimicrobial stewardship as the actions veterinarians take individually and as a profession to preserve the effectiveness and availability of antimicrobial drugs through conscientious oversight and responsible medical decision-making while safeguarding animal, public, and environmental health. For more information about stewardship visit these websites: AVMA Antimicrobial Stewardship The University of Minnesota Antimicrobial Resistance and Stewardship Initiative (ARSI) |
Interrelationship of Antimicrobial Resistance in Animals, Humans, and the Environment.
Antimicrobial drugs are used to treat, control, or prevent infections in people, animals, and plants.
- Treatment of disease: administering antimicrobial drugs to treat sick animals/humans/plants.
- Control of disease: disease is present in a percentage of a group (e.g., people attending the same school; a herd of cattle; or an orchard of fruit trees) and antibiotics are administered to the group to decrease disease spread while clinically ill individuals are treated.
- Prevention of disease: no individuals are currently exhibiting clinical signs of disease, but there is a known disease risk present. In other words, disease is likely to occur if antibiotics are not administered. Antibiotics are administered to prevent infection.
- Growth promotion / feed efficiency: With the implementation of the Veterinary Feed Directive in the United States on January 1, 2017, medically important antimicrobials are no longer allowed to be used for growth promotion or feed efficiency. For other antimicrobial drugs (e.g., ionophores, which have no use in human medicine), with approval from the US Food and Drug Administration (FDA) for this use category, the antimicrobials are administered to animals, usually in feed, to increase growth rates and improve feed efficiency of the animals.
While there are over 100 known individual antibiotics, the majority of these come from under 10 drug classes (the main classes of antibiotics are penicillins, cephalosporins, macrolides, fluoroquinolones, sulfonamides, tetracyclines, and aminoglycosides). Not surprisingly, there is overlap in the use of these antibiotic drug types among human, animal, and plant sectors. For example, streptomycin is used in all health sectors—human medicine, animal medicine, and plant agriculture—to treat or prevent infections. It follows that the problem of antimicrobial resistance exists in all sectors.
Interrelationship of Antimicrobial Resistance in Animals, Humans, and the Environment.
Antimicrobial drugs are used to treat, control, or prevent infections in people, animals, and plants (See Definitions box below).
- Treatment of disease: administering antimicrobial drugs to treat sick animals/humans/plants.
- Control of disease: disease is present in a percentage of a group (e.g., people attending the same school; a herd of cattle; or an orchard of fruit trees) and antibiotics are administered to the group to decrease disease spread while clinically ill individuals are treated.
- Prevention of disease: no individuals are currently exhibiting clinical signs of disease, but there is a known disease risk present. In other words, disease is likely to occur if antibiotics are not administered. Antibiotics are administered to prevent infection.
- Growth promotion / feed efficiency: With the implementation of the Veterinary Feed Directive in the United States on January 1, 2017, medically important antimicrobials are no longer allowed to be used for growth promotion or feed efficiency. For other antimicrobial drugs (e.g., ionophores, which have no use in human medicine), with approval from the US Food and Drug Administration (FDA) for this use category, the antimicrobials are administered to animals, usually in feed, to increase growth rates and improve feed efficiency of the animals.
While there are over 100 known individual antibiotics, the majority of these come from under 10 drug classes (the main classes of antibiotics are penicillins, cephalosporins, macrolides, fluoroquinolones, sulfonamides, tetracyclines, and aminoglycosides). Not surprisingly, there is overlap in the use of these antibiotic drug types among human, animal, and plant sectors. For example, streptomycin is used in all health sectors—human medicine, animal medicine, and plant agriculture—to treat or prevent infections. It follows that the problem of antimicrobial resistance exists in all sectors.
Infections with resistant organisms are difficult to treat, frequently requiring costly and sometimes toxic alternatives. Although some people and animals have a higher risk for such infections (e.g., the very young or very old and those with weakened immune systems), the risk of antibiotic-resistant infections cannot be completely avoided (see Risk box below).
|
Hazard vs. Risk:
A hazard is something with the potential to cause harm (e.g., a multidrug resistant organism that is capable of infecting and causing disease in humans, animals, and/or plants). Risk is the likelihood of occurrence and the magnitude of consequences of a specified hazard being realized (e.g., the probability of an animal becoming infected with and/or developing disease from a multidrug resistant organism). Risk Factor: something that increases risk. RISK FACTORS FOR INFECTION WITH RESISTANT ORGANISMS The very young, the very old, and those with weakened immune systems are more likely to become infected. Examples of these groups include children under 5 years of age, cats and dogs under 6 months of age, people over 65, pregnant women and animals, people with HIV infection, cats with FIV infection, or individuals with autoimmune diseases. Additional risk factors include long hospital stays, indwelling catheters, and poor healthcare hygiene (e.g., failure of caretakers to wash their hands). |
The direction of spread of AMR and AMR genes is not always completely understood and spurs continued debate. For example, a greater diversity of resistance phenotypes in human-associated organisms (Mather et al, 2011) may mean that humans pose a greater risk to animals than animals do to humans. And while the use of antibiotics in plant agriculture has resulted in the development of resistant plant pathogens, evidence of direct adverse human health effects is lacking (Stockwell 2012). Continued work is needed to understand these complex relationships and to promote antimicrobial stewardship.
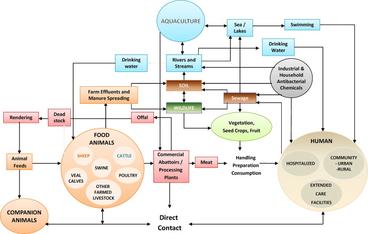
Figure legend: The interconnected web of potential exposures to antimicrobial resistant organisms from multiple sources highlights the importance of addressing the problem of AMR with a One Health approach. All those who use antimicrobial drugs or who may rely on antimicrobial drugs to treat, control, or prevent potential infections have a vested interest in keeping antimicrobial drugs effective.
To check your understanding of the material to this point - take this Quiz. Link to Quiz
Animals and the Spread of Antimicrobial Drug Resistance through Different Environments
Animals and the Spread of Antimicrobial Drug Resistance through Different Environments
Animals can serve as vehicles (or fomites), reservoirs, and direct disseminators (see definitions below) of some drug-resistant bacterial strains and AMR genes, with potential impacts on other animals, humans, and the environment.
- Vehicle: (with regard to vehicle-borne transmission) an inanimate object or material that can transmit an infectious agent – also called a “fomite.” Vehicles become contaminated with the infectious agent that has the potential to multiply or develop in or on the vehicle. The vehicle then contacts a susceptible host where it may be ingested, touch the skin, or be introduced internally during surgery or medical treatment. Some examples of such vehicles include cooking or eating utensils, bedding, clothing, toys, surgical or medical instruments (e.g., catheters), or dressings. Water, food, drinks (e.g., milk), and biological products (e.g., blood, serum, plasma, tissues or organs) can also be vehicles.
- Reservoir: any animal, person, plant, soil, substance—or combination of these—in which the infectious agent normally lives. The infectious agent must depend on the reservoir for its survival and must be able to multiply there. The infectious substance is transmitted from a reservoir to a susceptible host.
- Direct Disseminator: someone or something that can spread an infectious agent widely via direct transmission. Direct transmission involves immediate transfer of the infectious agent into a host’s body (e.g., through touching, biting, or sneezing/droplet spread, etc).
Animal production practices have evolved over the years to meet the animal-based protein needs of the growing human population. Disease prevention, husbandry, genetics, and nutrition have all greatly improved the efficiency of food animal production. To some extent, the mass production of animals was made possible by the availability of antibiotics for livestock and poultry. Although antibiotic use has benefited the food animal industry’s efforts to provide affordable animal protein for a burgeoning human population, the use of antibiotics in food production also contributes to the emergence and spread of important AMR microorganisms and to environmental contamination with antibiotics and their residues (FAO 2016).
The issue of AMR is much larger than antimicrobial drug use in animal agriculture alone. The movement of animals and animal-related products can also spread antimicrobial resistance. Within-country animal movement and international trade may contribute to the national and global spread of drug-resistant microbes. For example:
- In the United States (US), the movement and sale of puppies through a national pet store chain resulted in a multistate outbreak of multidrug-resistant Campylobacter infections in humans (CDC 2018).
- Multiple foodborne outbreaks associated with animal products have been recorded in the US. These outbreaks frequently spread to multiple states given the movement of products in the current food supply chain. Some of the causative pathogens have been resistant to one or more antimicrobial drugs (e.g., see CDC Reports of Selected Salmonella Outbreak Investigations).
- In Europe, trading non-heat-treated animal products, calves, and parent and grandparent poultry stock have been implicated in spreading Salmonella (Forshell, 2006).
Antibiotics administered to animals can reach the environment (e.g., soil) through direct application of animal manure used as agricultural fertilizer or through indirect routes like landfills (which can contain disposed medications, medical waste, or treated animal carcasses). Some agricultural antibiotic resistant organisms, such as Salmonella, have also been detected in dust from animal pens or barns (McEachran 2015). Soil and water contamination may occur through surface water run-off, driftage through wind, or leaching into the soil surface or groundwater. Antibiotics used for aquaculture may directly contaminate the aquatic environment, particularly when pens are placed in natural seawaters (Serrano 2005; Zou 2011). [For more information about antibiotics in aquaculture, see the Aquaculture Module.]
Animal feed and pet foods are additional vehicles for disease transmission and AMR spread (see Animal Feed sidebar), and their international trade continues to increase. Humans and animals also may be exposed to multi-drug resistant (MDR) organisms through direct contact with contaminated animal feed/pet food, pet treats, nutritional supplements, or animals and animal environments that have become infected or contaminated from these feed sources. Animals exposed to contaminated feed sources may be symptomatic or asymptomatic and can subsequently shed pathogens into their environments through their feces.
|
|
Connections Between Humans and Animals
Connections Between Humans and Animals
Animals are closely linked to humans through companionship (e.g., pet dogs and cats), work (e.g., police and service animals), recreation (e.g., horses), and food provision (e.g. meat, milk, eggs, honey). Animals also contribute to household products (e.g., clothes, cosmetics, art supplies) and to the growth of plant-based food sources (e.g., via fertilizers). Additionally, because humans and animals share the same general environment, they may share bacteria directly (through direct contact) or indirectly (through food products or the environment).
Molecular and epidemiological studies have shown that some AMR microorganisms in human populations can increase AMR problems in the animal population and vice versa. For example, methicillin-resistant Staphylococcus aureus (MRSA) is one of the most important hospital-acquired infections (see Definitions box below) in people. While most MRSA found in animals involves opportunistic infections (see Definitions box below) or transient colonization by strains of human origin, studies have shown that one risk factor for MRSA infections in humans is close contact with domestic animals that are more likely to circulate some MRSA strains (such as horses, pigs, cattle, dogs, and cats) (Lee 2003; Wulf et al 2006 & 2007; Anderson et al 2008; Khanna et al 2008; van Loo et al 2007; Weese 2010). Furthermore, research in animal species suggests that antimicrobial use may predispose hosts to MRSA infections. It is important to emphasize that in pet animals, most genotyping studies indicate that humans actually serve as the major source of MRSA (Weese 2010). [See Companion Animal Pyoderma module.]
Other interesting MRSA facts
- MRSA colonization in horses is typically transient, but opportunistic joint, skin, and deeper tissue infections are known to occur. Certain MRSA strains are believed to be endemic in horse populations in some regions, with colonization rates up to 43 percent on some farms (Weese, 2010). Strains isolated from horses include horse-adapted human strains and the ST398 MRSA strain typically found in livestock.
- Studies demonstrate relatively high levels of MRSA colonization in pigs and pig farmers, but rare clinical infections. Although the predominant strain in pigs (ST398) is believed to have evolved in swine, human clones have also been isolated, suggesting that transmission between pigs and humans may be bi-directional and initial strains actually may have originated in humans (Price et al 2012). In addition, a study in Denmark demonstrated that approximately one-third of ST398-associated skin and soft tissue infections occurred in people with no pig or other livestock contact, a finding that suggests community spread (Larsen, 2017).
- Although cattle contact has been associated with MRSA ST398 spread in humans, most studies have shown a low prevalence of MRSA isolation from cattle (Weese, 2010).
|
DEFINITIONS: Hospital-Acquired Infections (HAIs): also called nosocomial infections. Infections that patients get while being treated in a hospital. Healthcare-acquired or healthcare-associated infections: infections that patients get while receiving treatment for medical or surgical conditions in all care settings (e.g., hospitals, ambulatory clinics, rehabilitation facilities, outpatient care and long-term care facilities). Opportunistic infections: infections caused by pathogens (bacteria, viruses, fungi, or protozoa) that take advantage of an opportunity not normally available, such as a host with a weakened immune system, an altered microbiota (such as a disrupted gut flora), or breached integumentary barriers. Many of these pathogens do not cause disease in a healthy host that has a normal immune system. Source: https://en.wikipedia.org/wiki/Opportunistic_infection Multidrug resistant drug (MDR) organisms: organisms with acquired non-susceptibility to three or more classes of antimicrobial drug (refer to the Microbiology Module) |
Zoonotic Multidrug Resistant Outbreaks
Overall, animal-associated MDR pathogens represent a small, but important proportion of all MDR pathogens affecting humans. Healthcare-associated infections are a more common source of MDR pathogens in humans than animal-associated MDR pathogens (Chatterjee et al, 2018). Table 1 below summarizes some animal-associated outbreaks, illustrating the potential impact of animal-associated MDR pathogens.
Table 1. Sampling of outbreaks of zoonotic MDR bacterial infections
|
Organism |
Animal species |
Source |
Outbreak |
Year(s) |
Reference |
|
Salmonella Typhimurium |
Cats (humane society) |
Direct Contact |
7 people |
1999 |
Umber, 2009 |
|
Salmonella Typhimurium |
Pet rats, mice & hamsters |
Direct Contact and Contaminated Environments |
28 people 13 pet stores 10 states |
2003-2004 |
Swanson, 2007 |
|
Salmonella Java |
Tropical fish aquariums |
Direct Contact |
18 people |
2003-2004 |
Kirk, 2006 |
|
Salmonella Poona, S. IIIb 61:i:z53, and S. Sandiego |
Pet turtles |
Direct Contact |
4 outbreaks 133 people 26 states |
2015-2016 |
CDC, 2016 |
|
Salmonella Heidelberg |
Dairy calves |
Direct Contact |
54 people 15 states |
2015-2017 |
CDC, 2017 |
|
Campylobacter |
Puppies (pet store) |
Direct Contact |
113 people 17 states |
2016-2018 |
CDC, 2018 |
|
Salmonella Reading |
Poultry – Raw Turkey |
Foodborne and other potential direct contact |
358 people 42 states |
2017-2019 |
CDC, 2019 |
Medically Important Antibiotics
A primary focus in the fight against AMR is to maintain effective antibiotic drugs for use when needed to treat serious infections. Medically important antibiotics that are determined to be important for treating serious illnesses in humans are prioritized.
|
MEDICALLY IMPORTANT ANTIBIOTICS Follow these links to see the various classifications of antimicrobial drugs according to their importance to human and veterinary health:
|
In response to the growing threat of AMR, the United States issued a National Action Plan for Combating Antibiotic-Resistant Bacteria in 2015 (The White House, 2015), which has since been updated with a five year plan for 2020-2025 (Federal Task Force on Combating Antibiotic-Resistant Bacteria, 2020). One of the goals of the initial plan was to “eliminate the use of medically-important antibiotics for growth promotion in food-producing animals and bring other agricultural uses of antibiotics, for treatment, control, and prevention of disease, under veterinary oversight.”
The Food and Drug Administration (FDA) adopted measures to restrict the use of medically important antimicrobial drugs in food-producing animals, including tetracyclines, penicillins, and sulfonamides. The Guidance for Industry (GFI) 209, GFI 213, and the Veterinary Feed Directive Final Rule became effective on January 1, 2017. Medically important antibiotics may no longer be used for growth promotion and feed efficiency purposes in food animal production in the United States. Their use is now restricted to disease prevention, treatment, and control and requires a veterinarian’s prescription. These restrictions also apply to apiculture, placing veterinarians in the position of prescribing antibiotics for honeybee colonies. [See the Honey Bees Module.]
from atlantic-bees.com
Antibiotics for Plants and Humans
Antibiotics for Plants and Humans
Antibiotic use in plant agriculture remains an important and controversial topic. Antibiotics have been used in plant agriculture since the 1950s and are still used to prevent devastating bacterial diseases (See Quick Fact below) (Stockwell 2012). Without treatment to prevent bacterial outbreaks, the plant agriculture industry can experience significant losses. While there are restrictions for antibiotic application in the US plant agriculture industry, antibiotic resistant organisms and AMR genes have been found in the soil of treated orchards and on or within fruit from antibiotic-treated trees (Donato et al, 2010; Granastein et al, 2014). Antibiotics, antibiotic-resistant organisms, and AMR genes are known to be present in the natural environment even without obvious application of antibiotics via human agricultural practices (Durso et al, 2016). The complexities and knowledge gaps associated with agroecosystems and AMR (See AMR systems map) is an area with significant potential for One Health research and collaboration (Durso and Cook, 2018).
|
QUICK FACT: Two of the most prominent and most studied antibiotics used in plant agriculture are streptomycin and oxytetracycline, which are both used in the US to manage the plant bacterial diseases fire blight (Erwinia amylovora) and bacterial spot (Xanthomonas arboricola pv. pruni). |
The Impact of Antimicrobial Resistance on the Environment
The Impact of Antimicrobial Resistance on the Environment
An area of One Health concern is the transmission and accumulation of antibiotics and their residues (breakdown products) in the environment (see AMR Systems Map – Animals and the Environment page 5). Antibiotic use in human and animal health and agriculture can contribute to the growing problem of aquatic and terrestrial antibiotic contamination of the environment (See Contamination Sidebar). A fraction of the dose of an antimicrobial drug given to humans and animals can be excreted into the environment through the natural process of drug elimination (see Pharmacology module), even after passing through wastewater treatment plants as these plants are not designed to filter or eliminate antimicrobial drugs from treated water. Depending on the drug and pharmacokinetic factors, up to 30 - 90% of the parent drug and its active and inactive metabolites could be excreted in the urine or feces of treated people and animals.
|
SIDEBAR: CONTAMINATION Antibiotics that have been reported in ground and surface water include macrolides, sulfonamides, tetracycline, chloramphenicol, chlortetracycline, sulfamethazine, lincomycin, trimethoprim, sulfadimethoxine and sulfamethazine. A national survey of wastewater contaminants found that the veterinary and human antibiotic sulfamethoxazole was found in 23% of the 47 groundwater sites tested across the United States and is one of the most frequently detected chemical compounds in wastewater (Barnes et al 2008). A large proportion of aquatic antibiotic contamination is thought to be from human antibiotic usage like hospital effluents and municipal sewage and wastewater that eventually end up in the environment (Kummerer 2002). Studies worldwide have provided evidence of several antibiotics in the soil at concentrations reaching as high as one milligram per kilogram of soil. Examples include oxytetracycline and sulfachlorpyridazine (Kay et al 2004), sulfamethazine and chlortetracycline (Aust et al 2008). |
Anactive antibiotic drug can survive for an extended period of time in the environment. Survival will depend on its physical-chemical properties (molecular structure, size, shape, solubility, and hydrophobicity), prevailing climatic conditions, soil or water types, and other environmental factors (Kemper 2008). Antibiotic concentrations in the natural environment gradually decrease through their dilution in water and adsorption to clay minerals in soil, but their biological impact may persist for longer periods and affect many types of non-targeted environmental microorganisms (Sengelov et al 2003; Martinez 2008). Low concentrations of antibiotics, as are typically found in soil and water, may exert selection pressure on environmental bacteria, enabling the selection of resistant environmental microorganisms. They may also foster the exchange of resistance genes, helping to create a “bank” of transferable drug resistance genes (see Microbiology module).
Although the overall ecological impacts of residual antibiotics in the environment are largely unknown, it can be presumed that antibiotics that accumulate over time may have profound effects on some ecosystems. For example, antibiotics can be toxic to plants and animals, which could have disruptive ripple effects up the food chain. Antimicrobial drugs can inhibit plant growth and development by disrupting their local bacteria population and by causing inhibition of germination, root growth, or shoot growth (Brian 1957). Additionally, antimicrobial drugs can kill and disrupt reproduction in aquatic organisms low on the food chain, such as freshwater crustaceans (Wollenberger et al 2000; Migliore et al 1997). While high doses of drugs are more likely to be toxic, it has now been shown that low doses of antibiotics can stimulate plant growth and enhance their biological functions (Agathokleous et al, 2018), which also could have disruptive effects. For example, from a public and environmental health standpoint, flourishing plants and possible antibiotic residue accumulation within plant tissues could lead to both underestimation of and ongoing antibiotic exposure risks to individuals exposed to affected plants.
Risks of Antimicrobial Manufacturing and other Industrial Practices
In addition to the risks posed by individuals excreting antimicrobials into the environment, there is also concern for the risks posed by antimicrobial drug manufacturing. Pollution during the production phase can exacerbate this problem. During drug manufacturing, untreated waste products containing high levels of end products or active ingredients may be discharged into water courses (Lübbert et al 2017). Some experts argue that this point in the manufacturing process may pose particular risk for the spread of drug resistance in the environment because the concentrations of antimicrobial drugs may be orders of magnitude higher than concentrations at other sewage sites.
The Impact of Anticmicrobial Resistance on Animal Health
The Impact of Antimicrobial Resistance on Animal Health
Increased morbidity and mortality
Although the burden of AMR on human health has been widely studied and recorded, the impact of AMR on animal health is not as well defined. Nevertheless, as the examples below indicate, drug-resistant pathogens also pose a threat to animal health and welfare.
Companion animals
In companion and recreational animals, like dogs, cats, and horses, infections with antimicrobial-resistant pathogens may cause prolonged illness and significantly increased treatment costs. If an owner is financially constrained, the sequelae to these infections may result in patient euthanasia. An extreme example is a dog whose methicillin-resistant Staphylococcus pseudintermedius (MSRP) infection required treatment with linezolid and exceeded $25,000 (Bengtsson 2014). In another example, a large animal hospital in Sweden experienced an outbreak of MDR Salmonella Newport, which affected 61 animals, including 54 horses; the case fatality rate was 36 percent (Bengtsson 2014). As drug resistance spreads, veterinarians may increasingly face the ethical dilemma of whether or not to use on their animal patients critically important antimicrobial drugs (as designated for humans - see medically important antibiotics section in this module).
Livestock
Brachyspira hyodysenteriae infection in pigs demonstrates the impact of AMR on livestock health and welfare. This bacterial pathogen causes dysentery and can result in prolonged illness, poor growth, increased mortality, and profound economic loss. As resistance to tylosin and lincomycin has become widespread in this pathogen, pleuromutilin antibiotics, which inhibit bacteria protein synthesis, have come into greater use. As a consequence, Brachyspira hyodysenteriae resistance to this drug class has emerged, presenting serious challenges to control this disease globally.
The Impact of Antimicrobial Resistance on Human Health
The Impact of Antimicrobial Resistance on Human Health
Antibiotics can be lifesaving and are among the most commonly prescribed drugs in human medicine. In the United States, it has been estimated that the costs of treating AMR infections in humans is $2.2 billion annually (Thorpe et al, 2018). The World Health Organization (WHO) states that:
“patients with infections caused by drug-resistant bacteria are at increased risk of worse clinical outcomes and death, and consume more health care resources than patients infected with non-resistant strains of the same bacteria. Without effective antimicrobials for prevention and treatment of infections, medical procedures such as organ transplantation, cancer chemotherapy, diabetes management and major surgery (for example, caesarean sections or hip replacements) become high risk. Antimicrobial resistance increases the cost of healthcare with lengthier stays in hospitals and more intensive care required [e.g., additional antibiotic treatment, more diagnostic tests, and more pain management]” (WHO Antimicrobial Resistance Fact Sheet, 2018).
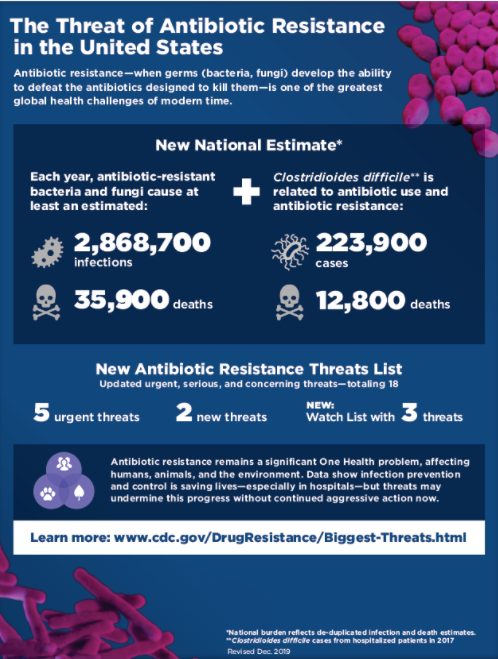
Higher case fatality rates occur in human patients infected with AMR organisms compared with those infected with antibiotic sensitive organisms (Helms et al, 2002). The CDC estimates that at least 35,900 people in the United States die from antibiotic resistant infections each year (see figure below; page 6 of the 2019 AR Threats Report).
Physicians rely on empirical treatments (See Definitions box below) when immediate therapy is necessary and cannot wait for laboratory testing. One concern with empirical therapy is using antibiotics that fail to treat infections because of resistance. For example, resistance to fluoroquinolones used to treat urinary tract infections is now widespread. In the 1980s, when these drugs were first introduced, resistance was virtually zero. Today, there are several countries where this antibiotic is now ineffective in more than half of patients (WHO Antimicrobial Resistance Fact Sheet, 2020). In some cases, microorganisms have developed resistance to “last resort” antibiotics that are usually reserved for the treatment of infections resistant to other antimicrobial drugs.
|
DEFINITIONS Antimicrobial use is often described with the following terms:
See the Pharmacology Module for discussion about preemptive use of antibiotics in veterinary medicine. A hospital-specific antibiogram is provided here as an example of a reference guide that clinicians can use when choosing appropriate antibiotics prior to obtaining culture and sensitivity results. |
Resistance to “last resort” antibiotics
- Resistance to carbapenem antibiotics (the last resort treatment for life-threatening infections caused by Klebsiella pneumoniae—an Enterobacteriaceae) has spread globally. K. pneumoniae is a major cause of hospital-acquired infections such as pneumonia, bloodstream infections, and infections in newborns and intensive-care unit patients. In some countries, because of this resistance, carbapenem antibiotics will not work in more than half of people treated for K. pneumoniae infections. (WHO Antimicrobial Resistance Fact Sheet, 2018).
- Colistin, or polymyxin E, has been used in animals and humans for decades and is one of the most commonly used antibiotics in animals in the European Union (EMA, 2016). In people, however, colistin is considered a last resort antibiotic because it is reserved for the treatment of multidrug resistant infections, in particular infections caused by carbapenem-resistant Enterobacteriaceae (CRE) (common Enterobacteriaceae are Klebsiella species and Escherichia coli [E. coli]). Colistin also can have serious side effects including kidney toxicity and respiratory distress. The first plasmid-mediated colistin resistance gene (mcr-1) was discovered in November 2015 and has since been detected in isolates from humans, animals, and the environment in over 30 countries (Yin, et al, 2017). A map on this page illustrates where mcr-1 has been reported in the U.S. Several additional mcr genes have been identified, and all seem to pose a serious challenge to the treatment of CRE. The mcr gene is carried on mobile genetic elements (e.g., plasmids), which are easily transmitted between bacteria and may contain other resistance genes.
- Enterococci are common gut microbes for warm-blooded animals, including humans. However, these bacteria sometimes cause problematic hospital-acquired infections. Antibiotic use has contributed to the emergence of multiple antibiotic resistant genes in this organism. Vancomycin is considered the treatment of choice for these resistant organisms, so the emergence and subsequent spread of vancomycin-resistant enterococci (VRE) became a significant public health concern. Before the 1990s, it was thought that VRE were present only in hospitals where the glycopeptide antibiotic vancomycin had been used for many years (Wegener, et al 1999). However, epidemiological and molecular studies have shown that the use of avoparcin, another glycopeptide antibiotic, as a growth promotant in farm animals resulted in carriage and dissemination of VRE (Wegener, et al 1999; Lu et al, 2004). Because of the potential public health concerns about resistance to vancomycin, avoparcin was banned in Denmark in 1995, in Germany in 1996, and eventually by all EU member states in 1997 (Wegener et al, 1999). Reductions in the prevalence of VRE in poultry, swine, and humans were reported in the subsequent years, although VRE linked to human infections in Europe has not disappeared (Wegener, 2003). Although vancomycin is still frequently used in the human hospital setting in the United States (US), avoparcin was never used in livestock and poultry in the US. This may be the reason why, in spite of the relatively high rates of VRE in US hospitals, there is less evidence of a community or zoonotic reservoir for VRE in this country (Ross, et al 2008).
CDC’s Biggest Threats
In 2013 the CDC published their first Antibiotic Resistance Threats in the United States report (2013 AR Threats Report). This report outlined 18 drug-resistant threats to the United States based on outbreak data and antimicrobial drug availability and research. The threats were categorized ranging from urgent, which require more monitoring and prevention activities, to serious, to concerning, which require less monitoring and prevention activities. The updated 2019 AR Threats Report also includes a Watch List with three resistance threats that have not yet spread widely in the United States but could become common without a continued aggressive approach. It is important to note that the majority of the MDR pathogens in the CDC’s AR Threats Report have no direct and limited indirect connection with antibiotic use in animals (e.g., drug-resistant Campylobacter and drug-resistant non-typhoidal Salmonella). Despite this, animal-associated MDR pathogens are an important part of the complex AMR challenge (Chatterjee A et al, 2018).
For more information about these drug resistant threats to the United States, please refer to the CDC’s Antimicrobial Resistance Biggest Threats and Data website.
On a global level, the World Health Organization (WHO), Food and Agriculture Organization of the United Nations (FAO), and the World Organisation for Animal Health (OIE) work together to promote best One Health practices to avoid the emergence and spread of AMR, including optimal antibiotic use in humans and animals. The WHO provides technical assistance for countries to develop national action plans which will strengthen their surveillance systems to prevent and manage antimicrobial resistance (WHO Global Action Plan on Antimicrobial Resistance 2015).
- In 2015, a global action plan on antimicrobial resistance was adopted by Member States at the 68th World Health Assembly. The goal of this global action plan is “to ensure, for as long as possible, continuity of successful treatment and prevention of infectious diseases with effective and safe medicines that are quality-assured, used in a responsible way, and accessible to all who need them” (WHO Global Action Plan 2015).
- The global action plan sets out five strategic objectives: (1) To improve awareness and understanding of antimicrobial resistance; (2) To strengthen knowledge through surveillance and research; (3) To reduce the incidence of infection; (4) To optimize the use of antimicrobial agents; and (5) To ensure sustainable investment in countering antimicrobial resistance
- In 2016, the United Nations General Assembly held a meeting to enhance national multi-sectoral efforts to combat antimicrobial resistance. In that same year, the FAO published its Action Plan on Antimicrobial Resistance 2016-2020 in support of the WHO action plan.
- In November 2017, the WHO published new guidelines for using medically important antimicrobials in food-producing animals (WHO, 2017).
- In 2018, the WHO, FAO, and OIE released a report analyzing countries’ responses to a self-assessment survey and describing the level of global progress on AMR (WHO, FAO, OIE 2018).
On a national level, many countries are developing national action plans. The US issued its first National Action Plan for Combating Antibiotic-Resistant Bacteria in 2015 (see the current plan via this link). Some countries have national agencies charged with monitoring antimicrobial usage and rates of AMR in food animals, food, and/or people. National and international agencies that are working to mitigate AMR include:
- National Antimicrobial Resistance Monitoring System (NARMS) in the US
- Canadian Integrated Program for Antimicrobial Resistance (CIPARS) in Canada
- Observatoire National de Epidémiologie de la Résistance Bactérienne aux Antibiotiques (ONERBA) in France
- The Danish Integrated Antimicrobial Resistance Monitoring and Research Programme (DANMAP) in Denmark
- Japanese Veterinary Antimicrobial Resistance Monitoring System in Japan
State organizations also support One Health approaches (e.g., see the Minnesota One Health Antibiotic Stewardship Collaborative) and antibiotic stewardship programs are becoming more common in veterinary and human medical settings alike. Antibiotic stewardship programs are meant to promote the appropriate use of antimicrobial drugs. While individual program specifics may vary (e.g., across disciplines or between hospitals) many of the core principles are the same. Examples of antimicrobial stewardship principles include (AVMA; CDC):
- Commit to stewardship and set goals to improve antibiotic use
- Advocate for a system of care to prevent common diseases
- Select and use antimicrobial drugs judiciously
- Track and evaluate antimicrobial drug use practices and resistance patterns
- Educate
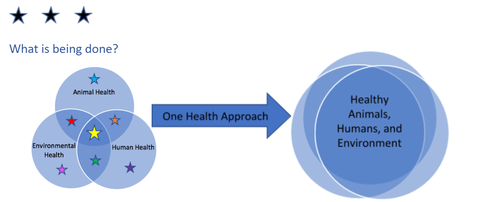
What more can be done?
There remains a need for coordinated action from the local level up to the global level. Antimicrobial resistance is a complex problem that affects all of society and is driven by many interconnected factors—improved coordinated action can better minimize the emergence and spread of AMR. From a global perspective, all countries should have national AMR action plans and invest more in AMR research to develop new antimicrobial medicines, vaccines, and diagnostic tools.
To prevent and control the spread of antibiotic resistance,
Individuals can:
- Only use antibiotics when prescribed by a certified health professional.
- Never demand antibiotics if your healthcare provider says you don’t need them.
- Always follow your healthcare provider’s advice when using antibiotics.
- Never share or use leftover antibiotics and always properly dispose of leftover antibiotics.
- Prevent infections by regularly washing hands, preparing food hygienically, avoiding close contact with sick people, covering nose and mouth when sneezing/coughing, practicing safer sex, and keeping vaccinations up to date.
Health professionals in human & veterinary medicine can:
- Prevent infections by ensuring your hands, instruments, and environment are clean.
- Only prescribe and dispense antibiotics when they are needed, according to current guidelines.
- Report antibiotic-resistant infections to surveillance teams.
- Talk to your patients/clients about how to take/give antibiotics correctly, antibiotic resistance, and the dangers of misuse.
- Talk to your patients/clients about preventing infections through vaccination, hand washing, and minimizing exposures.
- Develop/promote/support antibiotic stewardship efforts in hospitals, clinics, long-term care and shelter facilities.
Policymakers can:
- Ensure robust national,state, and/or local action plans to tackle antibiotic resistance are in place.
- Improve surveillance of antibiotic-resistant infections.
- Strengthen policies, programs, and implementation of infection prevention and control measures.
- Regulate and promote the appropriate use and disposal of quality medicines.
- Make information available on the impact of antibiotic resistance.
Industry and national, municipal, and trade organizations can:
- Increase awareness of the antibiotic resistance problem.
- Support development and implementation of antibiotic stewardship programs.
- Invest in research and development of new antibiotics, vaccines, diagnostics and other tools.
Agricultural producers can:
- Ensure adequate farm hygiene, worker safety, and biosecurity and biocontainment programs are in place.
- Ensure animal health programs are in place to reduce infections (e.g., vaccination).
- Work with appropriate professionals to ensure judicious antibiotic use.
The Grand Challenge
With antibiotic use comes selection pressure on bacterial populations. Research shows that gene swapping among bacteria does occur and that an expanding number of people, animals, and animal products transverse the globe much more quickly than ever before. In addition, there is a need for novel antibiotics to address the emergence of multidrug resistant organisms. Evidence of AMR persistence and spread in the environment combined with the risks posed by antibiotic resistant organisms presents a grand challenge. Today, a coordinated One Health approach is necessary to successfully preserve the use of antimicrobial drugs.
References
Agathokleous E, Kitao M, Calabrese EJ. 2018. Human and veterinary antibiotics induce hormesis in plants: Scientific and regulatory issues and an environmental perspective. Environ Int 120:489-495.
Anderson MEC, Lefebvre SL, Weese JS. 2008. Evaluation of prevalence and risk factors for methicillin-resistant Staphylococcus aureus colonization in veterinary personnel attending an international equine veterinary conference. Veterinary Microbiology 129:410-417.
Aust MO, Godlinski F, Travis GR, Hao X, McAllister TA, Leinweber P, Thiele-Bruhn S. 2008. Distribution of sulfamethazine, chlortetracycline and tylosin in manure and soil of Canadian feedlots after subtherapeutic use in cattle. Environmental Pollution. 156:1243-1251.
AVMA. One Health – What is One Health? 2018
Barnes KK, Kolpin DW, Furlong ET, Zaugg SD, Meyer MT, Barber LB. A national reconnaissance of pharmaceuticals and other organic wastewater contaminants in the United States—I) Groundwater. Sci. Total Environ. 2008, 402(2-3), 192–200.
Behravesh, C. B., Ferraro, A., Deasy, M., Dato, V., Moll, M., Sandt, C., ... & Urdaneta, V. 2010. Human Salmonella infections linked to contaminated dry dog and cat food, 2006–2008. Pediatrics, 126(3), 477-483.
Bengtsson B, Greko C. Antibiotic resistance--consequences for animal health, welfare, and food production. Ups J Med Sci. 2014 May;119(2):96-102. doi: 10.3109/03009734.2014.901445. Epub 2014 Mar 28.
Brian PW. 1957. Effects of antibiotics on plants. Annual Review of Plant Physiology. 8:413-426.
Centers for Disease Control and Prevention. 2017. Multistate Outbreak of Multidrug-Resistant Salmonella Heidelberg Infections Linked to Contact with Dairy Bull Calves (Final Update). Accessed Online February 2018. https://www.cdc.gov/salmonella/heidelberg-11-16/index.html
Centers for Disease Control and Prevention. 2017. One Health Basics.
Chatterjee A, Modarai M, Naylor NR, Boyd SE, Atun R, Barlow J, Holmes AH, Johnson A, Robotham JV. 2018. Quantifying drivers of antibiotic resistance in humans: a systematic review,
Lancet Infect Dis. Aug 29. pii: S1473-3099(18)30296-2. doi: 10.1016/S1473-3099(18)30296-2.
Donato JJ, Moe LA, Converse BJ, Smart KD, Berklein FC, McManus PS, Handelsman J. 2010.
Metagenomic analysis of apple orchard soil reveals antibiotic resistance genes encoding predicted bifunctional proteins. Appl Environ Microbiol. 76(13):4396-401. doi: 10.1128/AEM.01763-09.
Durso LM, Cook KL. 2018. One Health and Antibiotic Resistance in Agroecosystems.
Ecohealth. doi: 10.1007/s10393-018-1324-7. [Epub ahead of print]
Durso LM, Wedin DA, Gilley JE, Miller DN, Marx DB. 2016. Assessment of Selected Antibiotic Resistances in Ungrazed Native Nebraska Prairie Soils. J Environ Qual. 45(2):454-62. doi: 10.2134/jeq2015.06.0280.
Farias, L. F. P., Oliveira, C. J. B., Medardus, J. J., Molla, B. Z., Wolfe, B. A., & Gebreyes, W. A. 2015. Phenotypic and genotypic characterization of Salmonella enterica in captive wildlife and exotic animal species in Ohio, USA. Zoonoses and public health, 62(6), 438-444.
FAO. 2016. Drivers, dynamics and epidemiology of antimicrobial resistance in animal production.
Forshell, L.P. and Wierup, M., 2006. Salmonella contamination: a significant challenge to the global marketing of animal food products. Rev. sci. tech. Off. int. Epiz, 25(2), pp.541-554.
Helms M, Vastrup P, Gerner-Smidt P, and Molbak K. 2002. Excess Mortality Associated with Antimicrobial Drug-Resistant Salmonella Typhimurium. Emerging Infectious Diseases. 8(5):490-495.
Kay P, Blackwell PA, Boxall, ABA. 2004. Fate of veterinary antibiotics in a macroporous tile drained clay soil. Environmental Toxicology and Chemistry 23:1136–1144.
Kemper N. 2008. Veterinary antibiotics in the aquatic and terrestrial environment. Ecological Indicators: 1-13.
Khanna T, Friendship R, Dewey C, Weese J. 2008. Methicillin resistant Staphylococcus aureus colonization in pigs and pig farmers. Veterinary Microbiology. 128:298-303.
Kirk, M., Combs, B. G., Musto, J., Mwanri, L., & Lightfoot, D. 2006. Multidrug resistant Salmonella Java infections acquired from tropical fish aquariums, Australia, 2003-04. Communicable diseases intelligence quarterly report, 30(2), 222.
Kummerer K. 2002. Drugs in the environment: emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources – a review. Chemosphere. 45:957-969.
Larsen J, Petersen A, Larsen AR, Sieber RN, et al. 2017. Emergence of livestock-associated methicillin-resistant Staphylococcus aureus bloodstream infections in Denmark. Clinical Infectious Diseases. 1;65(7):1072-1076. doi: 10.1093/cid/cix504.
Larsson DGJ, Andremont A, Bengtsson-Palme J, Brandt KK, et al. 2018. Critical knowledge gaps and research needs related to the environmental dimensions of antibiotic resistance. Environ Int. 117:132-138. doi: 10.1016/j.envint.2018.04.041.
Lee JH. 2003. Methicillin(Oxacillin)-resistantStaphylococcus aureus Strains Isolated from Major Food Animals and Their Potential Transmission to Humans. Applied and Environmental Microbiology. 69(11):6489-6494.
Li, X., Bethune, L. A., Jia, Y., Lovell, R. A., Proescholdt, T. A., Benz, S. A., ... & McChesney, D. G. 2012. Surveillance of Salmonella prevalence in animal feeds and characterization of the Salmonella isolates by serotyping and antimicrobial susceptibility. Foodborne pathogens and disease, 9(8), 692-698.
Lu K, Asano R, Davies J. 2004. Antimicrobial Resistance Gene delivery in Animal Feeds. Emerging Infectious Diseases. 10(4):679-683.
Lübbert C, Baars C, Dayakar A, Lippmann N, Rodloff AC, Kinzig M, Sörgel F. Environmental pollution with antimicrobial agents from bulk drug manufacturing industries in Hyderabad, South India, is associated with dissemination of extended-spectrum beta-lactamase and carbapenemase-producing pathogens. Infection. 2017 Aug;45(4):479-491. doi: 10.1007/s15010-017-1007-2. Epub 2017 Apr 26.
Martinez JL. 2008. Antibiotics and antibiotic resistance genes in natural environments. Science. 321:365-367.
Mather AE, Denwood MJ, Haydon DT, Matthews L, Mellor DJ, Coia JE, Brown DJ, Reid SW. 2011. The prevalences of Salmonella Genomic Island 1 variants in human and animal Salmonella Typhimurium DT104 are distinguishable using a Bayesian approach. PLoS One. 6(11):e27220. doi: 10.1371/journal.pone.0027220
McEachran AD, Blackwell BR, Hanson JD, Wooten KJ, Mayer GD, Cox SB, & Smith PN. 2015. Antibiotics, Bacteria, and Antibiotic Resistance Genes: Aerial Transport from Cattle Feed Yards via Particulate Matter. Environ Health Perspect, 123: 337-43
Migliore L, Civitareale C, Gianfranco B and Di Deupis GD. 1997. Toxicity of several important agricultural antibiotics to Artemia. Water Research 31(7):1801-1806.
Prescott. J. Veterinary Microbiology, Volume 171, Issues 3-4, 2014, 273-278
Price LB, Stegger M, Hasman H et al. 2012. Staphylococcus aureus CC398: Host Adaptation and Emergence of Methicillin Resistance in Livestock. MBio. 21:3(1). doi:10.1128/mBio.00305-11
Ross CB, Duke SE, Ziprin RL, Harvey RB, Hume ME, Poole TL, Scott EHM, Highfield LD, Alali WQ, Andrews K, Anderson RC and Nisbet DJ. 2008. Antibiotic and Disinfectant Susceptibility Profiles of Vancomycin-Resistant Enterococcus faecium (VRE) Isolated from Community Waste water in Texas. Bull Environ Contam Toxicol. 80:188–194
Sengelov G, Agerso Y, Hallig-Sorensen B, Baloda SB, Anderson JS and Jensen LB. 2003. Bacterial Antibiotic Resistance Levels in Danish Farmland as a Result of treatment with pig manure slurry. Environ. Int. 28:587-595
Serrano PH. 2005. Responsible use of antibiotics in aquaculture. FAO Fisheries technical Paper 469. Food and Agriculture Organization of the United Nations
Swanson, S. J., Snider, C., Braden, C. R., Boxrud, D., Wünschmann, A., Rudroff, J. A., ... & Smith, K. E. 2007. Multidrug-resistant Salmonella enterica serotype Typhimurium associated with pet rodents. New England Journal of Medicine, 356(1), 21-28.
Thorpe KE, Joski P, Johnston KJ. 2018. Antibiotic-resistant infection treatment costs have doubled since 2002, now exceeding $2 billion annually. Health Aff (Millwood). 37(4):662-669. doi: 10.1377/hlthaff.2017.1153. Epub 2018 Mar 21.
U.S. Food and Drug Administration. 2013. CVM Issues Assignment to Collect and Analyze Samples of Pet Foods, Pet Treats, and Pet Nutritional Supplements in Interstate Commerce in the United States for Salmonella. Accessed Online July 2017. https://www.fda.gov/AnimalVeterinary/Products/AnimalFoodFeeds/Contaminants/ucm348644.htm
Umber, J. K., & Bender, J. B. 2009. Pets and antimicrobial resistance. Veterinary Clinics of North America: Small Animal Practice, 39(2), 279-292.
van Loo I, Huijsdens X, Tiemersma E, de Neeling A, van de Sande-Bruinsma N, Beaujean D, Voss A and Kluytmans J. 2007. Emergence of Methicillin-Resistant Staphylococcus aureus of Animal Origin in Humans. Emerging Infectious Diseases 13(12):1834-1839.
Weese, J. S., & van Duijkeren, E. 2010. Methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in veterinary medicine. Veterinary microbiology, 140(3), 418-
Wegener HC. 2003. Antibiotics in animal feed and their role in resistance development. Current Opinion in Microbiology.6:439-445.
Wegener HC, Aarestrup FM, Jensen LB, Hammerum AM and Bager F. 1999. Use of Antimicrobial Growth Promoters in Food Animals and Enterococcus faecium Resistance to Therapeutic Antimicrobial Drugs in Europe. Emerging Infectious Diseases. 5(3):329-335.
Wellcome Trust. United Nations Foundation. Sustaining global action on antimicrobial Resistance. 2017.
The White House. 2015. FACT SHEET: Obama Administration Releases National Action Plan to Combat Antibiotic-Resistant Bacteria. Accessed Online July 2017. https://obamawhitehouse.archives.gov/the-press-office/2015/03/27/fact-sheet-obama-administration-releases-national-action-plan-combat-ant
White, D. G., Datta, A., McDermott, P., Friedman, S., Qaiyumi, S., Ayers, S., ... & Zhao, S. 2003. Antimicrobial susceptibility and genetic relatedness of Salmonella serovars isolated from animal-derived dog treats in the USA. Journal of Antimicrobial Chemotherapy, 52(5), 860-863.
WHO Antimicrobial Resistance Fact Sheet, 2020.
WHO. Global Action Plan. 2015.
Wollenberger L Halling-Sorensen B, Kusk KO. 2000. Acute and chronic toxicity of veterinary antibiotics to Daphnia magna. Chemosphere. 40:723-730.
Wulf M, Nes AV, Eikelenboom-Boskamp A, de Vries J, Melchers W, Klaassen C and Voss A. 2006. Methicillin-resistant Staphylococcus aureus in Veterinary Doctors and Students, the Netherlands. Emerging Infectious diseases. 12(12):1939-1941.
Wulf MWH, Sorum M, van Nes A, Skov R, Melchers WJG, Klaassen CHW and Voss A. 2007. Prevalence of methicillin-resistant Staphylococcus aureus among veterinarians: an international study. Clinical Microbiology of Infectious Diseases. 14:29-34.
Yin W, Li H, Shen Y, Liu Z, Wang S, Shen Z, Zhang R, Walsh TR, Shen J, Wang Y. 2017. Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. mBio 8:e00543-17.
Zou S, Xu W, Zhang R, Tang J, Chen Y, Zhang G. Occurrence and distribution of antibiotics in coastal water of the Bohai Bay, China: impacts of river discharge and aquaculture activities. Environ Pollut. 2011 Oct;159(10):2913-20. doi: 10.1016/j.envpol.2011.04.037. Epub 2011 May 14